Tylenol is one of the most recognized over-the-counter pain relievers in the world. Many people wonder who owns Tylenol today, especially since it has changed hands over the years. Understanding its ownership, history, and parent company gives insight into how this global brand operates and who controls it.
Key Takeaways
- Tylenol is fully owned and operated by Kenvue Inc., an independent consumer health company spun off from Johnson & Johnson in 2023.
- Kenvue’s largest shareholders are institutional investors, with Vanguard Group (10.8%), Vanguard Index Funds (9.9%), BlackRock/iShares (5.2%), and State Street/SPDR (5.2%) holding the most significant stakes.
- Insider holdings, including executives and board members, account for roughly 1.13%, aligning management interests with shareholders.
- Tylenol benefits from broad investor backing, including public and retail investors holding roughly 20% of Kenvue, allowing the brand to maintain financial stability, invest in innovation, and expand into pediatric, cold & flu, sinus, and international markets.
Tylenol Company Profile
Tylenol is a leading over-the-counter analgesic brand whose active ingredient is acetaminophen (paracetamol outside the U.S.). The Tylenol brand now belongs to Kenvue, which spun off from Johnson & Johnson’s consumer health division.
As of 2025, Tylenol remains one of Kenvue’s flagship brands. The name “Tylenol” is derived from a chemical shorthand (N-acetyl-para-aminophenol). The brand is widely recognized and used globally for pain relief, fever reduction, and symptom relief in colds, headaches, and more.
Company Details
Kenvue Inc. is a public company focused solely on consumer health. It is the legal owner and operator of Tylenol. Kenvue is headquartered in New Jersey, U.S., with incorporation in Delaware. It manages a portfolio of well-known health, wellness, and personal care brands, including Tylenol, Band-Aid, Neutrogena, Aveeno, Listerine, Johnson’s Baby, and others.
Kenvue was once part of Johnson & Johnson’s consumer health division but became independent through a spin-off in 2023.
Before the spin-off, Tylenol was under McNeil Consumer Healthcare (a subsidiary division of Johnson & Johnson). McNeil had long been responsible for manufacturing and branding Tylenol products globally.
Founders and Origin of Tylenol
The Tylenol brand traces back to McNeil Laboratories, which introduced Tylenol in 1955. The founder behind McNeil Laboratories was Robert L. McNeil Jr., a chemist whose company developed the product. Over time, McNeil became part of the Johnson & Johnson corporate structure.
Thus, while Tylenol does not have a single founder in today’s structure (it is a brand), its genesis is tied to McNeil Labs and the chemist Robert L. McNeil Jr.
Major Milestones
- 1879: Robert McNeil purchases a small pharmacy in Philadelphia for $167. This becomes the foundation of the McNeil family business, which later evolves into McNeil Laboratories.
- 1949: McNeil begins research into acetaminophen (paracetamol) as an alternative to aspirin.
- 1951: The U.S. Food and Drug Administration approves acetaminophen (paracetamol) for use.
- 1955: McNeil Laboratories introduces Tylenol Elixir for Children as a prescription medicine in the U.S.
- 1959: Johnson & Johnson acquires McNeil Laboratories, bringing Tylenol into the J&J portfolio.
- 1960 / 1961: Tylenol becomes available over the counter in the U.S. (i.e., no longer requiring a prescription). In 1961, McNeil moves into its Fort Washington, Pennsylvania, operations and expands consumer products.
- 1977: McNeil splits into two divisions: McNeil Medical Products (for prescription drugs) and McNeil Consumer Products (for over-the-counter and consumer health) under J&J.
- 1982: The Chicago Tylenol murders occur. Seven people die after ingesting cyanide-tainted Tylenol capsules. The crisis forces a massive recall (31 million bottles) and leads J&J to pioneer tamper-resistant packaging and safety standards.
- Late 1980s / 1990s: Under leadership such as James Burke (CEO of J&J from 1976 to 1989), J&J reestablishes Tylenol’s reputation, restores consumer trust, and applies the lessons from the 1982 crisis to its safety and marketing practices.
- 1991: McNeil licenses the brand Lactaid from Alan Kligerman to expand its consumer health portfolio.
- 2010: McNeil Consumer Healthcare announces a voluntary recall of over-the-counter children’s medicine products including Tylenol, citing manufacturing defects in a plant in Fort Washington.
- 2021 (November): Johnson & Johnson publicly announces its plan to spin off its consumer health business — including Tylenol — into a separate entity.
- 2022 (February / mid-year): Kenvue is established (legally incorporated) to house J&J’s consumer health brands.
- 2023 (May): Kenvue holds its initial public offering (IPO) for consumer health assets.
- 2023 (August 23): Kenvue becomes fully independent from Johnson & Johnson through an exchange offer and formal separation. Johnson & Johnson retains ~9.5 % stake at that point.
- 2024 (May): Johnson & Johnson announces intention to sell its remaining 9.5 % stake in Kenvue (~182.3 million shares), moving to fully exit consumer health ownership.
- 2025 (mid-year): Kenvue’s CEO, Thibaut Mongon, steps down amid a strategic review; Kirk Perry is appointed interim CEO.
- 2025 (June): Kenvue begins exploring divestiture of some underperforming skin health/beauty brands (e.g., Clean & Clear, Neostrata) to refocus on core health brands like Tylenol.
Who Owns Tylenol?
Tylenol is currently owned and marketed by Kenvue Inc., a standalone consumer health company that was spun off from Johnson & Johnson in 2023. Kenvue operates Tylenol as one of its flagship brands in the over-the-counter (OTC) medicine category.
Unlike in earlier decades, Tylenol is no longer directly under Johnson & Johnson, although Johnson & Johnson retained a minority shareholding for a short period after the spin-off.
By 2024, Johnson & Johnson had completely divested its stake, leaving Kenvue fully independent.
Tylenol remains one of Kenvue’s core revenue drivers, particularly in the U.S. and global OTC markets. In recent years, Kenvue has also introduced innovations such as drug-free supplements under the Tylenol brand to expand its footprint beyond traditional pain relief. The strong trust associated with Tylenol has made it one of the most valuable consumer healthcare assets in the company’s portfolio.
Tylenol Parent Company
The parent company of Tylenol today is Kenvue Inc. Kenvue manages a broad portfolio of health and wellness products that include Tylenol, Motrin, Band-Aid, Listerine, Neutrogena, Aveeno, Zyrtec, and Johnson’s Baby products.
The company is publicly traded and headquartered in New Jersey, United States. Its entire business model revolves around consumer healthcare, unlike Johnson & Johnson, which operates across pharmaceuticals, medical devices, and consumer products.
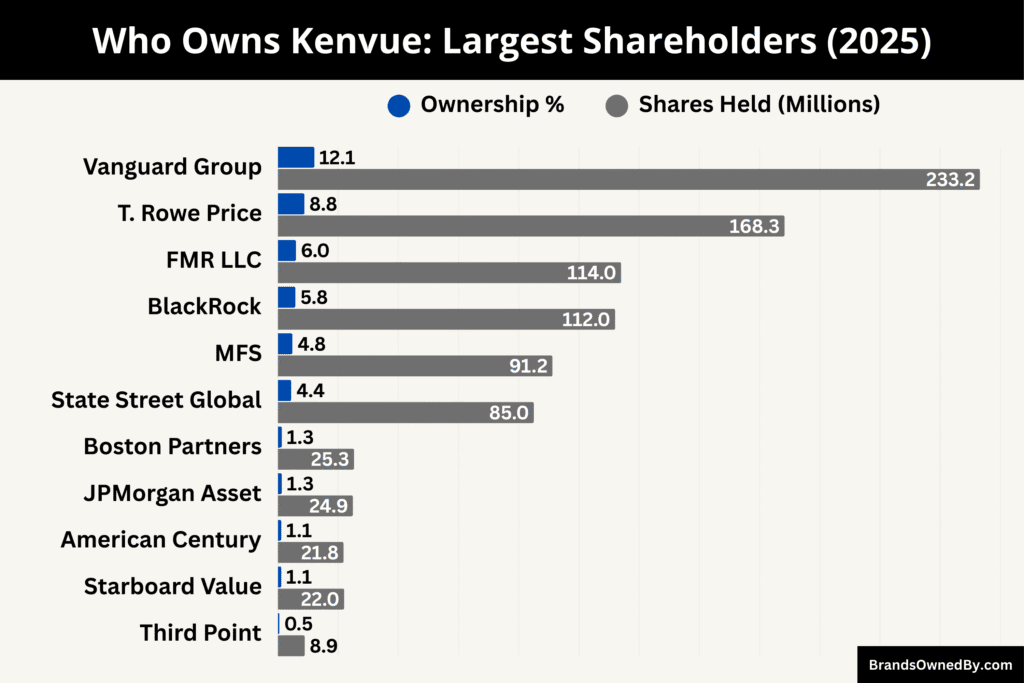
Below is a list of the major shareholders of Kenvue as of September 2025:
Vanguard Group, Inc.
Vanguard is Kenvue’s largest shareholder, holding 233.2 million shares, which represents 12.1% of the company. As a major index fund manager, Vanguard wields considerable influence over corporate governance and shareholder votes, even though it typically takes a passive approach to daily management.
T. Rowe Price Associates, Inc.
With 168.3 million shares (8.8%), T. Rowe Price is a key institutional investor in Kenvue. Known for its active fund management, the firm engages with management on long-term strategies, growth initiatives, and capital allocation decisions.
FMR LLC (Fidelity)
Fidelity holds 114 million shares, equating to 6.0% ownership. This mutual fund powerhouse represents a broad range of retail and retirement investors. Fidelity often emphasizes corporate transparency and steady performance in its engagement with companies.
BlackRock, Inc.
BlackRock owns 112 million shares (5.8%) of Kenvue. The company combines passive fund management with active stewardship, regularly voting on governance matters, ESG initiatives, and long-term strategy proposals.
Massachusetts Financial Services (MFS)
MFS controls 91.2 million shares, or 4.8% of the company. The firm focuses on consistent returns and long-term growth, often supporting operational efficiency and strategic stability through constructive dialogue with management.
State Street Global Advisors
State Street holds 85 million shares (4.4%), giving it significant influence in board votes and governance policies. The firm typically advocates for independence on the board, clear transparency, and accountability in corporate decisions.
Starboard Value
Starboard Value, an activist investor, owns 22 million shares (1.1%). Despite its smaller stake, the firm has a strong voice on the board, pushing for strategic initiatives, profitability improvements, and enhanced operational efficiency.
Boston Partners
Boston Partners holds 25.3 million shares (1.3%). As a long-term institutional investor, it prioritizes financial discipline and supports sustainable value creation alongside Kenvue’s management.
JPMorgan Asset Management
JPMorgan controls 24.9 million shares (1.3%), giving it a role in corporate governance. The firm typically aligns with management on strategic priorities while safeguarding shareholder interests.
American Century Investments
With 21.8 million shares (1.1%), American Century maintains a steady, supportive presence. The firm participates in proxy voting and promotes responsible corporate policies.
Third Point LLC
Third Point, an activist hedge fund, holds 8.9 million shares (0.5%). The fund engages with Kenvue to encourage portfolio optimization, capital efficiency, and stronger financial performance.
Johnson & Johnson (Exited)
Johnson & Johnson previously held 182 million shares, but as of 2024, it has fully divested its stake. While historically significant, J&J no longer has ownership or control over Kenvue.
Acquisition Insights and Details
The ownership journey of Tylenol began in 1955, when it was introduced by McNeil Laboratories, a company founded by Robert L. McNeil Jr. In 1959, Johnson & Johnson acquired McNeil Laboratories, bringing Tylenol into its growing consumer health division. This acquisition was significant, as it allowed Johnson & Johnson to become a dominant player in the OTC medicine market.
For decades, Tylenol was manufactured and marketed by McNeil Consumer Healthcare, a Johnson & Johnson subsidiary. The brand survived major challenges, including the 1982 Tylenol crisis, which reshaped packaging standards for OTC drugs worldwide. Despite controversies and recalls over the years, Tylenol remained one of the most trusted household names.
In 2023, Johnson & Johnson officially created Kenvue by spinning off its consumer health division, transferring Tylenol and other brands into this new entity. Kenvue launched as a publicly traded company through an initial public offering. Johnson & Johnson initially kept around a 9–10% stake but fully exited its position by mid-2024.
This finalized Tylenol’s transition from being part of Johnson & Johnson to becoming a central product under Kenvue’s independent ownership.
Who Manufactures Tylenol?
Tylenol is manufactured and marketed by Kenvue Inc., the consumer health company spun off from Johnson & Johnson in 2023. While Kenvue is the legal owner and brand manager, the manufacturing of Tylenol is handled through its internal production facilities, legacy divisions, and contract manufacturing partners to ensure global supply.
Kenvue’s Manufacturing Facilities
Kenvue operates several state-of-the-art manufacturing sites in the United States, including major facilities in:
- Fort Washington, Pennsylvania
- Las Piedras, Puerto Rico
- Springhouse, Pennsylvania.
These are the facilities where Tylenol products are formulated, packaged, and quality-tested. These facilities handle the full range of Tylenol products, including adult, pediatric, rapid-release gels, PM, cold & flu, and arthritis pain formulations.
Each facility is designed to meet stringent Good Manufacturing Practices (GMP) and FDA regulatory requirements, ensuring that every batch is safe, effective, and consistent.
The production lines include advanced tableting, capsule-filling, liquid suspension, and blister-packaging equipment, allowing the company to maintain high-volume output while minimizing errors.
Kenvue also invests in automated quality control systems, in-process testing, and environmental monitoring to maintain the integrity of all products. These facilities are regularly audited both internally and by regulatory agencies to ensure compliance with global standards, including Health Canada, the European Medicines Agency, and other international bodies.
Additionally, the company emphasizes sustainability and efficiency, incorporating energy-saving technologies, waste-reduction programs, and clean-room protocols in all manufacturing plants. This ensures that Tylenol production is not only high-quality but also environmentally responsible.
Contract Manufacturing Partners
In addition to internal production, Kenvue partners with contract manufacturing organizations (CMOs) for specific product lines and international markets. These partnerships allow Kenvue to meet global demand efficiently, scale production during peak seasons, and maintain supply chain flexibility. Products manufactured through CMOs undergo rigorous quality control to match the standards of Tylenol’s in-house production.
All Tylenol manufacturing, whether in Kenvue-owned facilities or CMOs, is subject to FDA, Health Canada, and other international regulatory standards. Each batch undergoes testing for purity, dosage accuracy, and safety. Kenvue’s manufacturing operations emphasize compliance, reliability, and traceability, which are critical to maintaining Tylenol’s reputation as a trusted OTC brand.
Global Manufacturing Footprint
Tylenol is produced in multiple regions globally, including North America, Europe, and select emerging markets, to serve both local and international supply needs. Regional production helps reduce shipping times, manage costs, and ensure timely availability in pharmacies, supermarkets, and online channels worldwide.
In Europe, Tylenol is produced in strategic facilities located in:
- Belgium
- France
- United Kingdom.
This allows Kenvue to supply the European market efficiently while meeting European Medicines Agency (EMA) standards. These facilities focus on both adult and pediatric formulations, including local packaging variations and compliance with EU labeling and regulatory requirements.
Tylenol is also produced in select emerging markets, including India, Brazil, and Mexico, where regional manufacturing helps reduce shipping times, manage costs, and improve market responsiveness. These facilities often use a combination of local production and contract manufacturing partners to scale capacity while adhering to local and international quality regulations.
Kenvue’s global production strategy ensures that Tylenol remains available year-round, even during periods of high demand, such as flu season or cold outbreaks. Regional manufacturing also allows the company to adapt quickly to local regulatory changes, optimize logistics, and minimize supply chain disruptions, maintaining Tylenol’s reputation as a reliable and accessible OTC pain-relief brand worldwide.
Who is the CEO of Tylenol?
Kenvue’s CEO oversees the entire consumer health portfolio, which includes Tylenol, Band-Aid, Neutrogena, and other major brands. The CEO plays a pivotal role in shaping strategy, managing operations, interfacing with shareholders, and steering innovation.
Current CEO (2025): Kirk Perry (Interim)
As of mid-2025, Kirk Perry is serving as interim CEO of Kenvue. He took over after the departure of the prior CEO during a period of strategic review and heightened activist investor involvement. Perry is expected to maintain continuity while guiding the company through portfolio rationalization, cost optimization, and increased pressure from shareholders to maximize value.
Role and Responsibilities
As CEO, Perry leads Kenvue’s strategic direction, operational oversight, and performance execution. He must balance demands from institutional investors, activist shareholders, and internal leadership teams. The responsibilities include:
- Setting and executing a long-term growth strategy for core brands like Tylenol
- Overseeing product innovation, research, and development
- Managing costs, supply chain, and global manufacturing
- Liaising with the board of directors and major shareholders
- Responding to activist proposals and governance pressures
- Ensuring regulatory compliance and brand reputation globally
Previous CEO: Thibaut Mongon
Before Kirk Perry’s interim appointment, Thibaut Mongon held the CEO role. Mongon led the spin-off from Johnson & Johnson and guided Kenvue through its early public years. His tenure focused on establishing the company’s independent identity, executing brand investments, and managing the initial transition period. Under his leadership, Kenvue navigated the challenges of separating from a large parent and maintaining continuity in consumer health operations.
Leadership Transition and Board Oversight
The board of directors was active in the leadership transition. Following activist investor pressures and performance reviews, the decision was made to replace or shift top leadership to better align with shareholder expectations. The board retains the authority to select permanent leadership and continues to evaluate performance against strategic benchmarks. During interim leadership, the board and senior executives collaborate closely to maintain momentum and stability.
CEO’s Influence on Tylenol
Although Tylenol is one of many brands under Kenvue, the CEO’s decisions apply directly to it. The CEO determines resource allocation, research priorities, marketing budgets, and pipeline investments. A shift in CEO or strategy can lead to changes in how aggressively Tylenol is developed, diversified, or leveraged in new product lines.
Tylenol Annual Revenue and Net Worth
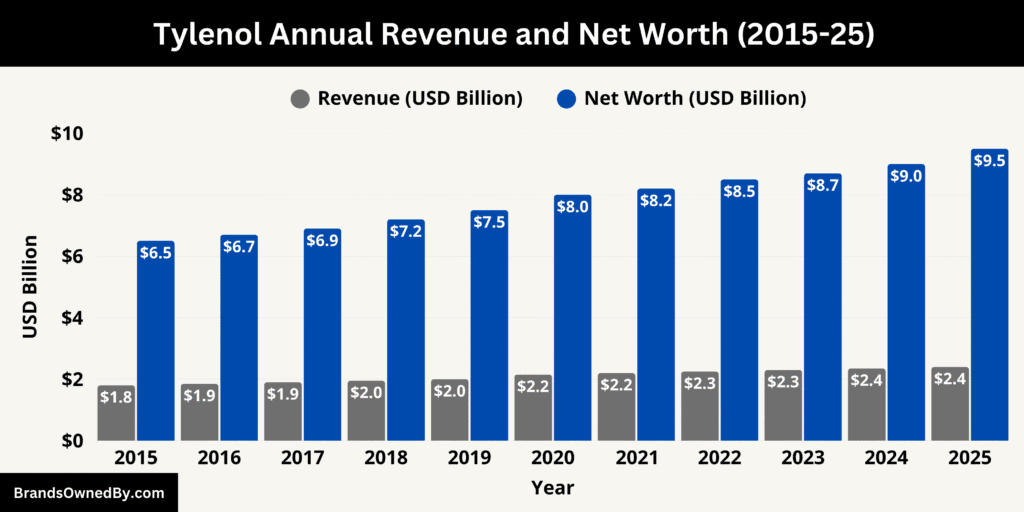
As of September 2025, the brand continues to generate strong annual revenue while maintaining significant market value. Its diverse product range—including adult, pediatric, cold & flu, and specialty formulations—supports consistent sales across multiple markets. The brand’s financial strength is reflected not only in its estimated annual revenue of around $2.4 billion but also in its substantial estimated net worth of $9.5 billion, making Tylenol one of the most recognized and profitable OTC healthcare brands globally.
Below is an overview of the revenue and net worth of Tylenol from 2015-25:
| Year | Estimated Revenue (USD Billion) | Estimated Brand Net Worth (USD Billion) | Notes |
|---|---|---|---|
| 2015 | 1.8 | 6.5 | Core adult pain relief products strong; pediatric line growing. |
| 2016 | 1.85 | 6.7 | Slight revenue growth; expanded retail and online distribution. |
| 2017 | 1.9 | 6.9 | Continued dominance in U.S. pain relief market; minor international expansion. |
| 2018 | 1.95 | 7.2 | Tylenol PM and cold & flu variants contribute to revenue growth. |
| 2019 | 2.0 | 7.5 | Pediatric products and Calpol bolster international sales. |
| 2020 | 2.15 | 8.0 | Pandemic-driven increase in OTC purchases; higher demand for pain and fever relief. |
| 2021 | 2.2 | 8.2 | Adult and pediatric lines remain strong; minor pricing adjustments. |
| 2022 | 2.25 | 8.5 | Continued expansion of Rapid Release and multi-symptom products. |
| 2023 | 2.3 | 8.7 | Post-Kenvue spin-off; brand remains central to Kenvue’s portfolio. |
| 2024 | 2.35 | 9.0 | Slight revenue growth from new formulations and digital marketing campaigns. |
| 2025 | 2.4 | 9.5 | Solid performance in U.S. and international markets; brand retains global leadership in pain relief. |
Annual Revenue
As of September 2025, Tylenol generates approximately $2.3–2.5 billion in annual global revenue. The United States remains the largest market, accounting for roughly 70–75% of total sales, followed by Canada, Europe, and select emerging markets.
Adult pain relief products like Tylenol Extra Strength, Regular Strength, and Rapid Release Gels contribute the majority of revenue. Pediatric variants and international versions such as Calpol add another significant portion. Seasonal and multi-symptom products, including Tylenol Cold & Flu and Tylenol PM, help smooth sales across quarters.
Revenue growth for Tylenol has been relatively steady, averaging 2–4% annually over the past few years, reflecting strong brand loyalty, wide distribution, and continued trust among consumers and healthcare professionals.
While the overall OTC pain-relief category faces competition from Advil, Aleve, and generic acetaminophen products, Tylenol maintains premium positioning and high margins.
Net Worth
The estimated net worth of the Tylenol brand—reflecting its market valuation as a standalone asset—is approximately $9.5 billion as of September 2025.
This estimate considers the brand’s revenue, market share, profitability, and strength of global recognition. Tylenol consistently ranks as one of the most valuable OTC pain-relief brands worldwide due to its trust, efficacy, and multi-segment product portfolio.
Market Position and Financial Significance
Tylenol is the largest individual revenue contributor within Kenvue’s pain-relief category. Its revenue accounts for roughly 15–17% of Kenvue’s total annual revenue, emphasizing its central role in the company’s financial health.
The brand’s strong profit margins are driven by premium pricing, extensive retail presence, and high repeat-purchase rates. International expansion, particularly through pediatric variants and multi-symptom formulas, continues to support incremental revenue growth.
Outlook
Tylenol’s financial strength remains resilient amid challenges like OTC competition, inflation, and generic substitutions. Brand extensions, reformulations, and targeted marketing campaigns in 2025 aim to maintain consumer trust and capture incremental market share.
Analysts forecast that Tylenol will continue generating steady revenue in the range of $2.3–2.7 billion annually while retaining its high brand value globally.
Brands Owned by Tylenol
Tylenol is not just a single product; it is a family of products that covers multiple segments: adult pain relief, pediatric care, cold & flu, sinus, arthritis, and specialized PM solutions. Each variant targets a specific consumer need while maintaining the brand’s core identity as a safe, effective, and trusted acetaminophen-based solution.
Here’s a list of the major brands owned by Tylenol as of 2025:
Tylenol Extra Strength
This is the core pain-relief formulation for adults. It contains higher doses of acetaminophen compared to regular Tylenol and is marketed for moderate to severe pain, including headaches, muscle aches, and back pain. Extra Strength remains one of the best-selling OTC painkillers in the United States.
Tylenol Regular Strength
Tylenol Regular Strength is designed for everyday mild-to-moderate pain relief. It has lower acetaminophen content than Extra Strength and is widely used for common headaches, minor aches, and fever reduction. It is positioned as a safe, everyday option for all ages.
Tylenol Rapid Release Gels
This product line features gel capsules formulated to dissolve quickly, allowing for faster pain relief. It appeals to consumers seeking immediate relief from headaches, migraines, or muscular pain. Rapid Release Gels have been a key innovation under the Tylenol brand.
Tylenol Arthritis Pain
Specifically designed for people with chronic joint pain or arthritis, this variant provides extended pain relief. It may include formulation adjustments that allow for longer-lasting effects while maintaining safety for long-term use. Tylenol Arthritis Pain is a major component of Tylenol’s adult-care sub-brand.
Tylenol PM
Tylenol PM combines acetaminophen with a mild sleep aid (diphenhydramine) to relieve nighttime pain while helping the user fall asleep. It is marketed for people with sleep disruption due to pain, making it a niche but important segment within the Tylenol family.
Tylenol Cold & Flu
This line extends Tylenol into multi-symptom relief for cold and flu sufferers. Products in this category include combinations of acetaminophen, decongestants, and antihistamines to target pain, fever, congestion, and other cold-related symptoms. It broadens Tylenol’s relevance beyond pure pain relief.
Tylenol Children’s
Tylenol Children’s products are acetaminophen-based formulations for infants, toddlers, and young children. This includes liquid suspensions, chewable tablets, and fever reducers. Tylenol Children’s is highly trusted among parents and pediatricians alike.
Tylenol Infants’ / Calpol (International)
In international markets, Tylenol is sometimes marketed as Calpol, particularly for infants and children. It contains age-appropriate acetaminophen formulations and is one of the leading pediatric brands in countries outside the U.S.
Tylenol Sinus
Tylenol Sinus products combine acetaminophen with decongestants to relieve sinus pressure, headaches, and congestion. This line positions Tylenol in the sinus-care segment and competes with other combination cold and sinus medications.
Tylenol Menstrual / PM Variants
Tylenol also offers formulations targeted for menstrual pain relief and specialized PM variants with a sleep aid. These products expand the brand into lifestyle-specific pain relief, reinforcing Tylenol’s presence in adult self-care.
Conclusion
Tylenol is owned by Johnson & Johnson through its consumer health spin-off Kenvue. It has a long history dating back to 1955 and remains one of the most popular pain relief brands in the world. With strong revenues, global recognition, and the backing of one of the most powerful healthcare companies, Tylenol continues to be a leader in the over-the-counter medicine market.
FAQs
Is Tylenol owned by Kenvue?
Yes. Tylenol is fully owned by Kenvue Inc., the consumer health company that spun off from Johnson & Johnson in 2023.
Who owns Tylenol products?
All Tylenol products are owned and marketed by Kenvue Inc., including adult, pediatric, cold & flu, and specialty formulations.
Who manufactures Tylenol?
Tylenol is manufactured by Kenvue’s in-house facilities in the United States, Europe, and select emerging markets, as well as through contract manufacturing partners.
Who owns the Tylenol brand?
The Tylenol brand is owned by Kenvue Inc., making it a flagship product in the company’s consumer health portfolio.
Who makes Tylenol products?
Tylenol products are made by Kenvue’s manufacturing facilities, including locations in Pennsylvania, Puerto Rico, and Texas, and through approved contract manufacturers globally.
Which company makes Tylenol?
Kenvue Inc. is the company that produces and markets all Tylenol products worldwide.
Why was Tylenol invented?
Tylenol was invented to provide a safe and effective pain reliever without the side effects associated with aspirin, particularly for reducing fever and relieving pain in adults and children.
When did Tylenol come out?
Tylenol was first introduced in 1955 as a prescription pain reliever and later became available over the counter.
Who is the founder of Tylenol?
Tylenol was developed by McNeil Laboratories, which later became part of Johnson & Johnson. The product’s creation is attributed to McNeil’s team of chemists and researchers in the 1950s.
Who owns Kenvue Company?
Kenvue is a publicly traded company. Its largest shareholders are institutional investors, including Vanguard Group (~10.8%), Vanguard Index Funds (~9.9%), BlackRock/iShares (~5.2%), and State Street/SPDR (~5.2%). Insiders and management hold about 1.13%, while other public investors hold the remainder.
What country is Tylenol made in?
Tylenol is primarily manufactured in the United States, including Pennsylvania, Puerto Rico, and Texas, with additional production in Europe (Belgium, France, UK) and select emerging markets such as India, Brazil, and Mexico.
Is Kenvue related to Johnson and Johnson?
Yes. Kenvue was spun off from Johnson & Johnson in 2023 as an independent consumer health company. Tylenol was part of J&J’s consumer health division prior to the spin-off.
Who makes Tylenol in the USA?
In the United States, Tylenol is manufactured by Kenvue’s U.S. facilities, including sites in Fort Washington, Springhouse (Pennsylvania), Las Piedras (Puerto Rico), and Fort Worth (Texas).
Who first made Tylenol?
Tylenol was first made by McNeil Laboratories in the 1950s as a prescription pain reliever.
What is the real name of Tylenol?
The generic name of Tylenol is acetaminophen (also called paracetamol in many countries). Tylenol is the brand name.
Is Tylenol the same as ibuprofen?
No. Tylenol contains acetaminophen, which works as a pain reliever and fever reducer, whereas ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that reduces pain, fever, and inflammation.
Is Tylenol paracetamol?
Yes. Tylenol is acetaminophen, which is known as paracetamol outside the United States and Canada.
What are Tylenol’s ingredients?
The active ingredient in Tylenol is acetaminophen. In addition, different formulations contain inactive ingredients such as binders, fillers, flavorings, sweeteners, and coatings, depending on whether it is a tablet, caplet, liquid suspension, chewable, or gel form.

