If you’ve ever wondered who owns Ozempic, the answer leads to one of the most influential pharmaceutical giants in the world — Novo Nordisk A/S. The Danish company is behind this breakthrough diabetes and weight-loss drug that has reshaped modern medicine. Understanding who owns Ozempic helps reveal how a single company transformed from an insulin maker into a global healthcare powerhouse.
Key Takeaways
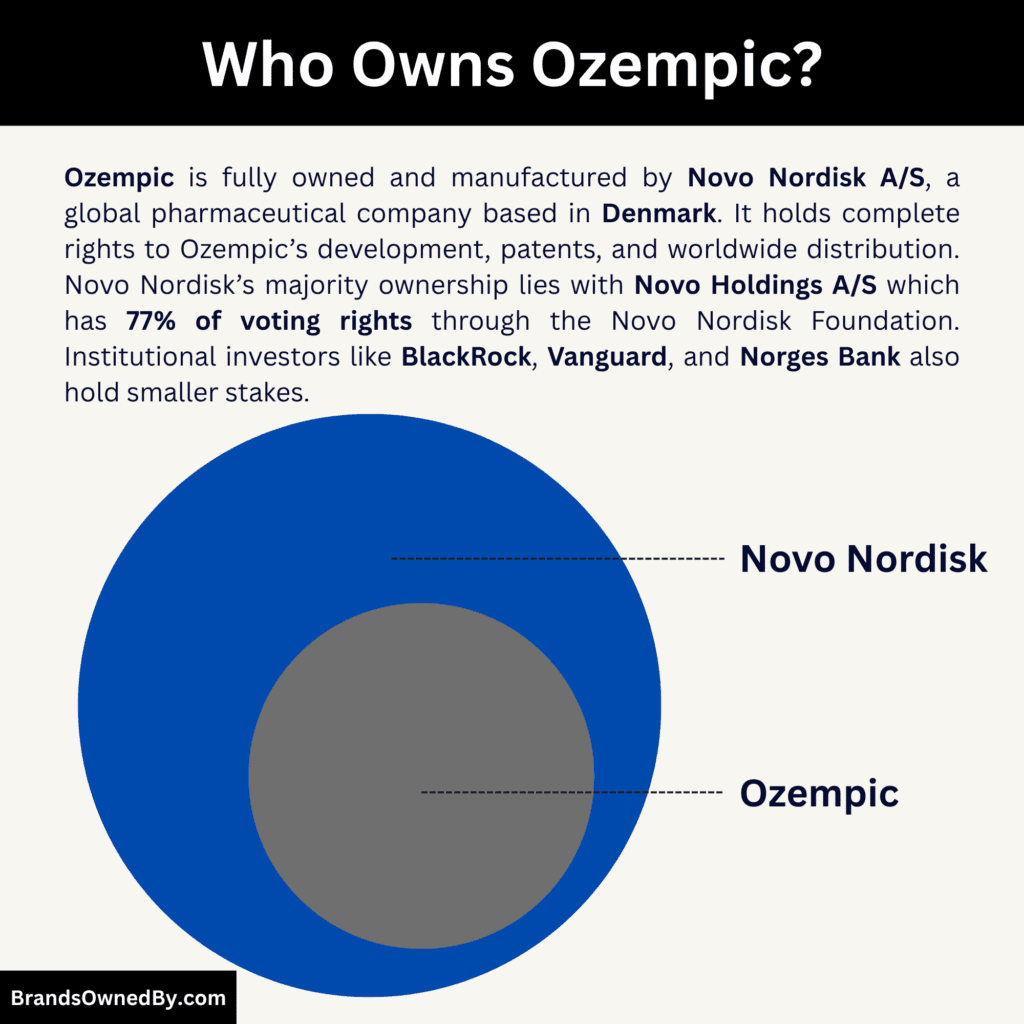
- Ozempic is owned and produced by Novo Nordisk A/S, a Danish pharmaceutical company that holds full rights to its development, patents, manufacturing, and global distribution. It is not a standalone company or co-owned by any other entity.
- Novo Nordisk is majority controlled by Novo Holdings A/S, which owns about 28.1% of total shares and 77.1% of voting rights through a dual-share structure. Novo Holdings is wholly owned by the Novo Nordisk Foundation, ensuring long-term control and reinvestment in scientific and healthcare innovation.
- Institutional investors like BlackRock (4.1%), Vanguard (3.2%), and Norges Bank Investment Management (1.8%) hold smaller economic stakes, giving them financial interest but limited governance influence.
- This ownership structure makes Ozempic’s control foundation-led and stability-focused, balancing public investment with strategic, long-term decision-making that prioritizes healthcare impact and sustained innovation.
Ozempic Overview
Ozempic is a prescription injectable medication that contains the active ingredient semaglutide. It is primarily used to help adults with type 2 diabetes control their blood sugar, in combination with diet and exercise.
In addition, Ozempic has been approved to lower the risk of major cardiovascular events such as heart attacks and strokes in patients with type 2 diabetes and established heart disease.
The U.S. FDA has also approved Ozempic to slow the progression of chronic kidney disease in people with diabetes. The drug belongs to a class known as GLP-1 receptor agonists and is administered once a week using a prefilled injection pen.
Ozempic is developed, manufactured, and marketed by Novo Nordisk A/S, a Danish multinational healthcare company headquartered in Bagsværd, Denmark.
The company specializes in treatments for diabetes, obesity, growth hormone disorders, and rare diseases. Novo Nordisk’s research and development facilities in Denmark played a central role in the creation of semaglutide, the molecule behind Ozempic.
As of 2025, the drug is sold globally and approved in more than 100 countries. Novo Nordisk continues to expand its production capacity to meet growing worldwide demand, especially as the popularity of GLP-1 therapies rises for both diabetes and weight management.
Founding and Origin
Ozempic itself doesn’t have a single individual founder, but its creation is the result of Novo Nordisk’s decades-long scientific research on GLP-1 hormones.
The foundation for this work was laid by scientists such as Mads Krogsgaard Thomsen, the company’s former Chief Scientific Officer, who oversaw the semaglutide development program.
The early concept of GLP-1 analogs originated in the 1990s when researchers began modifying natural GLP-1 to make it more stable and longer-lasting in the human body.
This work led to the development of liraglutide (approved in 2009) and later semaglutide — the compound that powers Ozempic.
The team of Danish researchers and global collaborators under Novo Nordisk’s leadership helped bring Ozempic from lab discovery to clinical success.
Major Milestones
- 1990s: Early research into GLP-1 analogs begins, focusing on glucose regulation and appetite suppression.
- 2001–2005: Novo Nordisk develops modified GLP-1 molecules with extended half-life suitable for weekly injection.
- 2012: Semaglutide, the active compound in Ozempic, enters pre-clinical development and early human trials.
- 2015: Phase III clinical trials begin under the SUSTAIN program, evaluating semaglutide for type 2 diabetes.
- 2017: The U.S. Food and Drug Administration (FDA) approves Ozempic for adults with type 2 diabetes.
- 2018–2019: Ozempic launches in Europe, Japan, and other global markets; real-world data confirm its cardiovascular benefits.
- 2021: New data highlight Ozempic’s ability to significantly reduce heart-related risks, prompting broader clinical recommendations.
- 2022: Demand for semaglutide-based treatments surges worldwide, leading to temporary supply shortages.
- 2023: Updated labeling in the U.S. and EU includes expanded cardiovascular risk reduction benefits.
- 2024: Ongoing studies show positive results for kidney protection in patients with type 2 diabetes and chronic kidney disease.
- 2025: The U.S. FDA officially approves Ozempic to reduce the risk of kidney failure and disease progression in adults with type 2 diabetes and chronic kidney disease. Ongoing global research explores its potential in metabolic and heart health beyond diabetes.
Who Owns Ozempic?
Ozempic is fully owned and produced by Novo Nordisk A/S, a Danish multinational pharmaceutical company. It is not a separate legal entity or subsidiary but a brand under Novo Nordisk’s portfolio of diabetes and metabolic disease treatments. All intellectual property rights, manufacturing licenses, and global marketing approvals for Ozempic are held directly by Novo Nordisk.
The drug’s active ingredient, semaglutide, was discovered and developed entirely in Novo Nordisk’s research laboratories. This means the company owns the complete patent, production process, and commercial rights to Ozempic across all global markets. The drug’s success has become one of Novo Nordisk’s most important assets, driving a large share of its revenue and market growth since its approval in 2017.
Parent Company: Novo Nordisk A/S
The parent company, Novo Nordisk A/S, is headquartered in Bagsværd, Denmark, and operates in more than 170 countries. Founded in 1923, the company has become one of the world’s largest pharmaceutical corporations, with a primary focus on diabetes, obesity, hemophilia, and hormone replacement therapies.
Novo Nordisk is publicly traded on the Nasdaq Copenhagen and has American Depositary Receipts (ADRs) listed on the New York Stock Exchange (NYSE: NVO). Despite being publicly listed, the company’s governance structure gives majority voting control to a single holding entity, ensuring long-term strategic stability.
The parent company’s success has been largely driven by its innovation in GLP-1 receptor agonist therapies, the class to which Ozempic belongs. Its other major products include Wegovy (for weight loss), Rybelsus (oral semaglutide), and NovoRapid (insulin aspart).
As of 2025, Novo Holdings A/S owns about 28.1% of Novo Nordisk’s total share capital and holds approximately 77.1% of the total voting rights. This majority voting power means Novo Holdings A/S effectively controls the strategic direction, long-term planning, and corporate governance of Novo Nordisk — and, by extension, Ozempic.
Institutional Shareholders
Alongside Novo Holdings, several major institutional investors own significant portions of Novo Nordisk’s shares, primarily the publicly traded “B-shares.” These shares grant financial ownership but limited voting control.
- BlackRock, Inc.: Holds around 4.1% of Novo Nordisk’s shares. As a global asset management firm, BlackRock is one of the largest institutional investors worldwide, offering financial rather than managerial influence.
- The Vanguard Group, Inc.: Owns approximately 3.2% of Novo Nordisk’s shares. Like BlackRock, Vanguard represents millions of investors through its mutual funds and ETFs, contributing to Novo Nordisk’s global investor base.
- Norges Bank Investment Management (the investment arm of Norway’s sovereign wealth fund): Holds roughly 1.8% of the company’s shares, reflecting its long-term investment focus on high-performing, sustainable companies.
- Other Institutional and Retail Shareholders: The remaining shares — around 60% of the total share capital — are widely held by institutional investors, pension funds, and individual retail shareholders globally. These investors benefit economically from Novo Nordisk’s growth, though strategic control remains with Novo Holdings.
Control and Governance
While many investors share in the profits generated by Ozempic, control of the drug’s future — from R&D investment to global expansion — lies with the Novo Nordisk Foundation via Novo Holdings A/S. This structure ensures that Ozempic’s development and production remain aligned with Novo Nordisk’s broader commitment to sustainable healthcare and innovation, rather than short-term market pressure.
Who Manufactures Ozempic?
Ozempic is part of Novo Nordisk’s expanding portfolio of GLP-1 (glucagon-like peptide-1) receptor agonists, which includes other major brands such as Wegovy (for weight management) and Rybelsus (an oral version of semaglutide). As of 2025, Novo Nordisk manufactures and distributes Ozempic in over 100 countries worldwide, with the U.S., U.K., Canada, and the European Union as major markets.
Novo Nordisk operates a global production network to meet the high demand for Ozempic. Manufacturing is carried out at multiple state-of-the-art facilities that adhere to strict regulatory standards such as Good Manufacturing Practice (GMP) and international quality certifications.
Key Ozempic manufacturing locations include:
- Denmark (Kalundborg and Hillerød): The core facilities for drug substance production and fill-finish operations. The Kalundborg site is one of the world’s largest insulin and peptide manufacturing plants.
- United States (Clayton, North Carolina): Novo Nordisk’s U.S. site handles large-scale production and packaging for the North American market.
- France (Chartres): A major European facility responsible for filling, assembly, and distribution across the EU and global markets.
- Brazil and China (new capacity expansions): Ongoing expansions to support regional demand and reduce global supply shortages.
These facilities ensure an uninterrupted global supply while maintaining the same quality and efficacy standards across all regions.
How Ozempic is Produced?
The manufacturing of Ozempic involves a highly specialized biotechnological process. The active ingredient, semaglutide, is produced using recombinant DNA technology. Novo Nordisk uses genetically engineered yeast cells to produce the semaglutide molecule, which mimics the human GLP-1 hormone that regulates blood sugar levels.
The process includes:
- Fermentation: Yeast cells are cultured in controlled bioreactors to produce semaglutide.
- Purification: The active ingredient is isolated and purified through advanced chromatography methods to ensure pharmaceutical-grade quality.
- Formulation: Semaglutide is mixed with excipients and stabilizers to create the injectable solution.
- Filling and Packaging: The solution is filled into Novo Nordisk’s proprietary FlexTouch® injection pens, ensuring precise dosing and patient safety.
- Quality Testing: Each batch undergoes rigorous testing for potency, sterility, and consistency before release.
This end-to-end process is managed under Novo Nordisk’s internal quality systems, with every production site regularly inspected by regulatory authorities such as the U.S. FDA, European Medicines Agency (EMA), and Health Canada.
Expansion and Production Capacity
The massive global demand for Ozempic and related semaglutide-based medicines has driven Novo Nordisk to significantly expand its production capacity. Between 2023 and 2025, the company invested billions of dollars to expand manufacturing capabilities:
- Catalent Acquisition (2024): Novo Nordisk acquired three Catalent manufacturing sites to boost fill-finish capacity for Ozempic and Wegovy pens.
- Kalundborg Expansion (2025): A new large-scale production line was added in Denmark to increase peptide synthesis output.
- North Carolina Expansion: A major U.S. facility expansion is ongoing to ensure faster delivery and better supply stability in the Americas.
These expansions help Novo Nordisk manage demand surges and prevent global shortages of Ozempic.
Quality and Regulatory Oversight
Every Ozempic batch undergoes multi-stage quality assurance and is tracked through a fully traceable supply chain. Novo Nordisk maintains compliance with all major regulatory agencies, including:
- U.S. Food and Drug Administration (FDA)
- European Medicines Agency (EMA)
- Health Canada
- Japan’s Pharmaceuticals and Medical Devices Agency (PMDA).
The company follows strict pharmacovigilance and post-marketing surveillance to monitor the drug’s performance and safety once it reaches patients.
Who is the CEO of Ozempic?
Ozempic, one of the world’s best-selling diabetes and chronic disease treatments, is produced and managed by Novo Nordisk A/S, a Danish pharmaceutical powerhouse. The company’s leadership — from its executive team to its board of directors — plays a direct role in steering Ozempic’s global strategy, expansion, and innovation. As of October 2025, Novo Nordisk has undergone major leadership and board changes, signaling a new era for the company and its flagship drug.
The CEO Leading Ozempic’s Future
As of August 2025, Maziar Mike Doustdar serves as the President and Chief Executive Officer (CEO) of Novo Nordisk. His appointment marked a historic shift — Doustdar became the first non-Danish CEO in the company’s 100-year history. Having joined Novo Nordisk decades ago in Vienna, he worked across international markets and corporate operations before taking the top role.
Under his leadership, Novo Nordisk has focused on making Ozempic and its related GLP-1 products more accessible worldwide. Doustdar is driving a global efficiency and restructuring plan aimed at streamlining operations and increasing production capacity to meet soaring demand. He is also prioritizing expansion into emerging markets and new indications, such as kidney and cardiovascular disease.
Doustdar’s leadership style is pragmatic and globally oriented — focusing not just on financial performance but also on long-term innovation, ethical distribution, and sustainable healthcare impact.
Leadership Style and Strategic Priorities
Doustdar has been clear about his focus on execution, efficiency, and global growth. He has identified that Novo Nordisk’s core strength lies in its diabetes and obesity therapies — including flagship products like Ozempic — and has signaled a shift toward faster decision-making, global expansion (particularly in emerging markets), and streamlined operations.
Soon after taking office, he initiated a transformation plan that includes headcount reduction and cost optimization to sharpen the company’s competitive edge in the GLP-1 and metabolic disease markets.
Compensation and Financial Standing
According to the company’s Remuneration Report for 2024, the structure of executive pay includes a fixed base salary, a short-term cash-based incentive, a long-term share-based incentive, pension contribution and other benefits.
While precise numbers for Doustdar’s total compensation as CEO in 2025 are not publicly disclosed, previous company disclosures show that the CEO’s base salary is aligned with senior executive peer benchmarks and subject to annual reviews.
One public note mentioned a CEO payout of about $1.5 million in a given year, though that figure does not necessarily reflect the full compensation package (which may include long-term incentives).
Doustdar’s personal shareholding in Novo Nordisk is small — about 0.002% of the company’s shares, valued at roughly $4.8 million.
The Role of the Board of Directors
The Board of Directors oversees Novo Nordisk’s strategic direction, ensuring that products like Ozempic continue to lead in science, safety, and global reach. The board approves company strategy, supervises executive decisions, and represents the interests of both the Novo Nordisk Foundation (the controlling shareholder) and public investors.
Because Ozempic generates a significant portion of Novo Nordisk’s revenue, the board’s policies directly influence its pricing, marketing, and regulatory strategies worldwide.
Major Board Changes in 2025
In October 2025, Novo Nordisk announced one of the most significant leadership shakeups in its history. A disagreement between the Novo Nordisk Foundation (the company’s controlling shareholder) and the existing board triggered a sweeping reorganization aimed at strengthening corporate agility.
As part of this change, Chairman Helge Lund and six independent directors announced they would step down at an extraordinary general meeting scheduled for November 14, 2025. The foundation nominated a new slate of directors to usher in a more proactive governance approach.
The proposed new leadership includes:
- Lars Rebien Sørensen, former CEO of Novo Nordisk (2000–2016), set to return as Chairman.
- Cees de Jong, experienced biopharmaceutical leader, to serve as Vice Chairman.
- Britt Meelby Jensen, Mikael Dolsten, Stephan Engels, and Helena Saxon as new board members.
Employee-elected directors and the CEO of Novo Holdings (the investment arm of the foundation) will remain in place to ensure continuity.
This shakeup reflects the foundation’s belief that the company needs a more agile, globally responsive board — one that can adapt quickly to the evolving pharmaceutical landscape and rising competition in GLP-1 therapies, the category that includes Ozempic.
What These Changes Mean for Ozempic
These leadership transitions come at a pivotal time. Global demand for Ozempic continues to surge, and the company is working to scale up production while fending off strong competition from rival drugs.
The new CEO and upcoming board are expected to prioritize:
- Expanding global production to prevent supply shortages.
- Accelerating innovation to stay ahead in the GLP-1 and metabolic therapy markets.
- Reinforcing ethical distribution and ensuring fair access across regions.
- Enhancing shareholder and foundation alignment for long-term growth and social responsibility.
This shift in leadership indicates a forward-thinking approach. With Mike Doustdar at the helm and Lars Rebien Sørensen expected to chair the renewed board, Novo Nordisk is positioning itself to strengthen Ozempic’s global dominance and sustain its reputation as a leader in diabetes and metabolic innovation.
Ozempic Annual Revenue and Net Worth
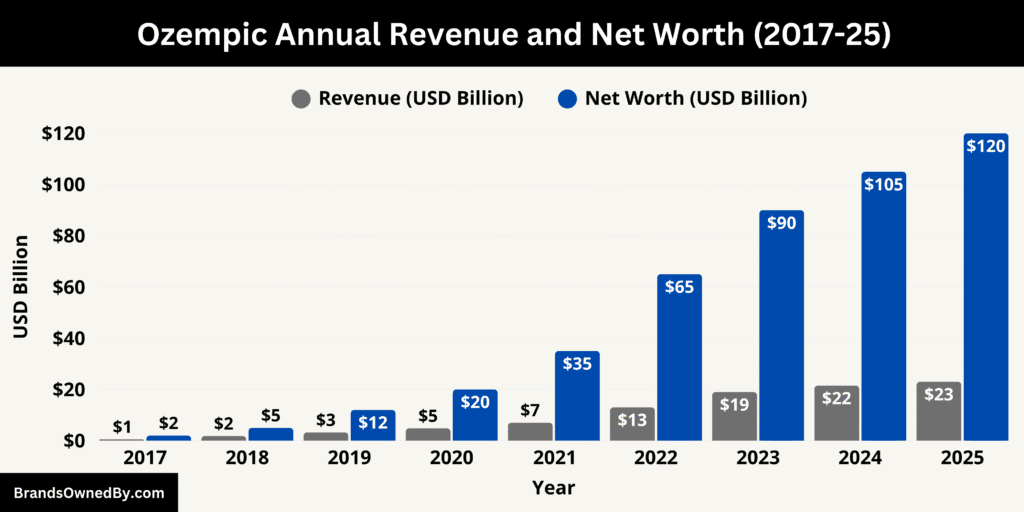
As of October 2025, Ozempic’s annual revenue stands at roughly $23–25 billion, cementing its position as the world’s leading GLP-1 therapy. It’s estimated brand valuation exceeds $120 billion, making it one of the most valuable pharmaceutical products ever developed.
Ozempic’s Annual Revenue in 2025
In 2025, Ozempic generated an estimated $23–25 billion in annual global revenue, representing over half of Novo Nordisk’s total pharmaceutical sales. This marks a sharp increase from 2024, when annual revenue was approximately $19 billion. The continued rise is fueled by the growing adoption of GLP-1 receptor agonists for both diabetes management and broader cardiometabolic health benefits.
The United States remains the largest market, contributing nearly two-thirds of Ozempic’s total global revenue. Europe follows, with strong uptake in Germany, France, and the United Kingdom. Growth in emerging markets, particularly in Asia and Latin America, has accelerated following expanded regulatory approvals and increased healthcare access programs.
A major growth driver in 2025 is the FDA’s expanded approval of Ozempic for patients with type 2 diabetes and chronic kidney disease. This new indication opened access to a much larger patient population, further solidifying the drug’s position as a multi-purpose therapy in chronic disease management.
Estimated Brand Valuation and Net Worth
While Ozempic is not a separate company and therefore has no formal net worth, its brand valuation as of 2025 is estimated to exceed $120 billion. This figure reflects the brand’s revenue-generating potential, patent protections, and future earnings outlook. It positions Ozempic among the most valuable pharmaceutical products ever created, rivaling historic blockbusters such as Humira, Lipitor, and Keytruda.
Analysts project that over the course of its patent lifespan, Ozempic could generate over $400 billion in total lifetime sales, given its dominance in type 2 diabetes and expanding use in cardiometabolic care. The drug’s strong pricing power, coupled with high patient retention, continues to support its exceptional financial performance.
Past and Future Revenue and Net Worth Analysis
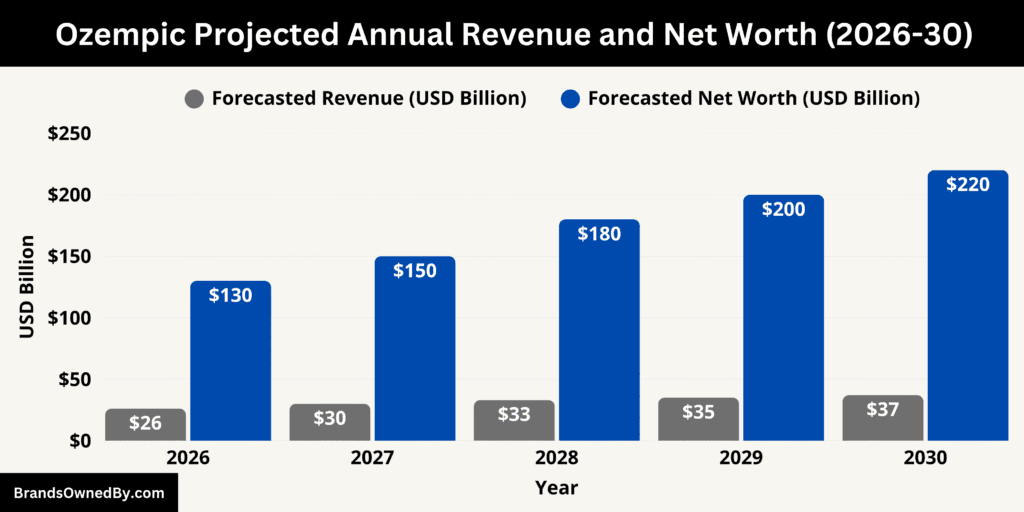
Over the last decade, Ozempic’s financial growth has been extraordinary. From zero revenue in 2015 to over $25 billion in 2025, the drug’s rise reflects a transformation in diabetes and metabolic care. Its brand valuation has grown proportionally, reaching over $120 billion, driven by its consistent sales growth, medical credibility, and global recognition.
The most significant revenue jumps occurred between 2020 and 2023, when demand outpaced production capacity. Novo Nordisk’s subsequent manufacturing expansion helped stabilize supply and sustain growth through 2025. With continuing approvals for new indications, Ozempic’s financial strength and brand dominance are expected to remain strong for the foreseeable future.
The projected revenue growth through 2030 reflects three major factors:
- Expanding indications (for example, kidney disease and cardiovascular risk)
- Increased global adoption (especially emerging markets)
- Continued dominance in the GLP-1 category.
However, the brand valuation estimates must also account for looming patent expirations and potential generic competition, which may dampen long-term upside.
By 2030, assuming the drug remains widely used and retains pricing power, Ozempic could be generating mid-thirty-billion-dollar annual sales and hold a brand valuation well over $200 billion under this scenario. These numbers underscore why it is regarded as one of the most commercially significant therapies of the decade.
Factors Behind Ozempic’s Financial Growth
The financial success of Ozempic is anchored in several interconnected factors.
First, the drug’s clinical profile is unmatched in its class, offering significant improvements in blood sugar control, cardiovascular outcomes, and kidney health. This scientific credibility has led to broad physician confidence and strong patient demand.
Second, Novo Nordisk’s global marketing and educational outreach have turned Ozempic into one of the most recognizable prescription brands in the world. Its presence in both medical and popular culture has fueled awareness and patient-driven inquiries.
Third, Novo Nordisk’s ongoing expansion of manufacturing capacity has alleviated past supply bottlenecks. Facilities in Denmark, the United States, and France have scaled up significantly since 2023, allowing for a steady supply and consistent revenue recognition throughout 2025.
Market Performance and Future Outlook
Ozempic’s financial trajectory in 2025 confirms its status as the top-selling diabetes medication worldwide.
Analysts expect moderate but steady revenue growth in the coming years as Novo Nordisk continues to expand access and maintain clinical leadership in GLP-1 therapies. However, increased competition from Eli Lilly’s GLP-1 drugs and other emerging biopharmaceutical competitors may begin to limit growth rates after 2026.
Patent protection for semaglutide, the active ingredient in Ozempic, is expected to remain in force until at least 2031 in major markets, safeguarding its profitability for much of the next decade. Even after patent expiration, the brand’s established reputation and physician trust are likely to sustain meaningful market share against future biosimilars.
Brands Owned by Ozempic
As of 2025, Ozempic’s extended brand ecosystem — encompassing Ozempic FlexTouch, Ozempic 2.0, Ozempic CKD, and the Digital Companion app — has transformed it from a single diabetes medication into a globally recognized healthcare platform built around patient outcomes, innovation, and trust.
Below is a list of the major brands owned by Ozempic as of October 2025:
| Brand / Entity Name | Type | Primary Purpose | Key Features and Details |
|---|---|---|---|
| Ozempic (Injectable Semaglutide) | Core Drug Brand | Treats type 2 diabetes; reduces cardiovascular and kidney risks | Once-weekly injectable GLP-1 receptor agonist that improves blood sugar control and reduces heart and kidney complications. Available in multiple doses: 0.25 mg, 0.5 mg, 1 mg, and 2 mg. |
| Ozempic FlexTouch | Medical Device (Injection Pen) | Simplifies and automates drug delivery | Spring-loaded pen design for smooth injection, accurate dosing, and easy use. Eliminates need for vials and syringes. Integral part of Ozempic’s patient-friendly image. |
| Ozempic 2.0 (High-Dose Formulation) | Drug Line Extension | Provides stronger glycemic and cardiovascular control | A 2 mg version of Ozempic designed for patients needing greater metabolic management. Offers enhanced clinical outcomes and improved kidney protection. |
| Ozempic CKD (Chronic Kidney Disease Indication) | Specialized Therapy Extension | Targets diabetes with chronic kidney disease | Expanded indication for patients with type 2 diabetes and CKD. Proven to slow kidney damage progression while maintaining glycemic control and heart protection. |
| Ozempic Ready-to-Use Kits | Patient Support Product | Helps new patients start Ozempic safely and confidently | Includes FlexTouch pen, needles, instructions, and educational materials. Branded for global distribution through hospitals, clinics, and pharmacies. |
| Ozempic Digital Companion App | Digital Health Platform | Monitors treatment progress and supports adherence | Mobile app offering reminders, glucose tracking, and integration with fitness devices. Provides educational materials and lifestyle guidance. |
| Ozempic Cardiometabolic Program | Medical Research & Education Initiative | Expands clinical understanding of semaglutide’s benefits | A global clinical and physician outreach program studying Ozempic’s role in heart, kidney, and metabolic health. Strengthens brand credibility in science and care. |
| Ozempic Lifestyle Series | Educational & Wellness Campaign | Promotes healthy living for diabetes patients | Branded awareness campaign featuring workshops, online events, and partnerships with health organizations to support patient lifestyle changes. |
| Ozempic Pen Plus Program | Device and Supply Initiative | Expands access to pen accessories and refills | Provides continuous supply of FlexTouch refills and needle replacements for regular users. Improves adherence and convenience for patients on long-term therapy. |
| Ozempic Global Access Program | Social Responsibility Initiative | Expands affordable access in developing markets | Works with local governments and global health agencies to make Ozempic more accessible in low- and middle-income countries. Reinforces the brand’s global commitment. |
Ozempic (Injectable Semaglutide)
The main product in the Ozempic brand portfolio is the once-weekly injectable formulation of semaglutide, designed for adults with type 2 diabetes. It helps control blood sugar levels, reduces the risk of heart attack and stroke, and, since its latest approval, slows the progression of chronic kidney disease. Ozempic is available in multiple dosage strengths — 0.25 mg, 0.5 mg, 1 mg, and 2 mg — allowing physicians to tailor treatment for each patient.
The brand’s success is rooted in its combination of strong clinical results and patient convenience. Unlike daily insulin injections, Ozempic requires only one weekly dose, making it one of the easiest diabetes treatments to maintain long-term. The brand’s identity emphasizes confidence, control, and medical reliability, which has helped Ozempic become the leading injectable diabetes treatment worldwide.
Ozempic FlexTouch
The Ozempic FlexTouch is the signature injection pen used to deliver the drug. It’s an integral part of the brand — not just a device but a key component of Ozempic’s identity. The FlexTouch system was designed to simplify injections for patients, particularly those new to self-administration.
The pen’s mechanism is spring-assisted, allowing for smooth and effortless dosing with minimal force. It has become widely recognized for its ergonomic design, single-click functionality, and easy-to-read dosage indicators. Patients appreciate the convenience of pre-filled pens, which eliminate the need for vials and syringes. Novo Nordisk continuously updates the FlexTouch design to improve grip, safety, and dose precision, aligning it with Ozempic’s commitment to patient-centered care.
Ozempic 2.0 (High-Dose Formulation)
Ozempic 2.0 refers to the expanded high-dose version of the original Ozempic formula. The 2 mg dose was introduced to provide stronger glycemic control for patients whose diabetes required a more intensive approach. This version also offers enhanced cardiovascular and kidney protection, backed by extended clinical trial data.
Ozempic 2.0 is particularly popular among patients transitioning from insulin therapy or those with more advanced metabolic complications. It also represents the brand’s strategic effort to remain the first choice among physicians in the competitive GLP-1 treatment space. Novo Nordisk markets this version under the same Ozempic name to maintain unified brand strength and recognition, ensuring that patients see it as part of the same trusted therapy line.
Ozempic CKD (Chronic Kidney Disease Indication)
Ozempic CKD is a branded therapeutic extension that focuses on adults with type 2 diabetes and chronic kidney disease. Approved in 2025, this version of Ozempic is formulated for patients at risk of kidney failure or worsening renal function. It’s the first GLP-1-based treatment to receive official recognition for kidney protection, making it a milestone in diabetes care.
Unlike the standard Ozempic treatment, Ozempic CKD targets both metabolic and renal health. Clinical data show that it helps slow kidney damage progression while maintaining blood sugar control and cardiovascular safety. This extension represents Novo Nordisk’s growing emphasis on treating the interconnected nature of chronic diseases. The CKD indication is now included in Ozempic’s global labeling, but it is branded separately in some markets for clarity.
Ozempic Ready-to-Use Kits
The Ozempic Ready-to-Use Kits are comprehensive starter packages for new patients. Each kit includes the Ozempic FlexTouch pen, pre-filled cartridges, disposable needles, usage instructions, and educational materials. These kits are provided through pharmacies, hospitals, and healthcare programs to simplify the initiation of treatment.
The kits also feature patient-friendly guides explaining how to manage diabetes effectively while using Ozempic. The packaging maintains consistent branding across regions, using the same color scheme and visual identity that has become synonymous with the Ozempic name. This approach enhances patient confidence and reinforces brand loyalty.
Ozempic Digital Companion App
The Ozempic Digital Companion App is Novo Nordisk’s patient support platform integrated directly into the Ozempic ecosystem. It allows users to track their blood sugar levels, schedule weekly injection reminders, and monitor weight and diet progress. The app also includes educational resources about diabetes management and connects patients with healthcare professionals for follow-ups.
Branded under the Ozempic name, the app helps patients maintain adherence and understand their treatment outcomes. It syncs with smart glucose monitors and fitness devices, offering real-time feedback. The digital platform reflects Novo Nordisk’s shift toward connected healthcare and personalized treatment — making Ozempic not just a medicine, but a digital health experience.
Ozempic Cardiometabolic Program
The Ozempic Cardiometabolic Program represents the research and educational branch of the Ozempic brand. It’s a long-term clinical and medical outreach initiative focused on exploring semaglutide’s benefits in cardiovascular and metabolic health. The program includes global clinical trials, real-world evidence studies, and physician training efforts under the Ozempic brand name.
This initiative strengthens Ozempic’s medical credibility by demonstrating its effectiveness beyond blood sugar control. Through this program, the brand actively collaborates with healthcare institutions, promoting awareness of how GLP-1 therapies can prevent complications related to diabetes, heart disease, and kidney failure.
Ozempic Lifestyle Series
The Ozempic Lifestyle Series is a branded educational campaign under the Ozempic name that aims to empower patients through awareness, nutrition advice, and lifestyle support. It’s not a product but a key marketing and health initiative tied to the brand’s mission. The series includes community workshops, online webinars, and partnerships with dietitians and healthcare organizations.
By positioning Ozempic as not just a prescription drug but a lifestyle-enabling treatment, Novo Nordisk reinforces the emotional and motivational appeal of the brand. This series contributes significantly to Ozempic’s reputation as a trusted, holistic solution for long-term diabetes and metabolic health management.
Final Thoughts
The answer to who owns Ozempic lies with Novo Nordisk A/S — a company built on decades of research, innovation, and purpose. Its ownership through the Novo Nordisk Foundation ensures long-term growth and reinvestment in medical progress. The story of who owns Ozempic isn’t just about corporate control; it’s about how Novo Nordisk continues to lead the future of healthcare through science, commitment, and global impact.
FAQs
Who is the owner of Ozempic?
Ozempic is owned by Novo Nordisk A/S, a Danish multinational pharmaceutical company. Novo Nordisk holds full rights to the development, manufacturing, and global distribution of Ozempic. It is not co-owned or licensed to any other company.
Who owns Ozempic and Wegovy?
Both Ozempic and Wegovy are owned and produced by Novo Nordisk A/S. They contain the same active ingredient, semaglutide, but are marketed under different brand names — Ozempic for type 2 diabetes and Wegovy for chronic weight management.
Who makes Wegovy and Ozempic?
Novo Nordisk A/S makes both Wegovy and Ozempic. The company oversees every stage of production, from research and formulation to manufacturing, packaging, and distribution, across its global network of facilities in Denmark, the United States, and Europe.
Which company owns Ozempic?
The pharmaceutical company that owns Ozempic is Novo Nordisk A/S. It holds all trademarks, patents, and regulatory approvals for the drug.
Who owns Ozempic Ozempic1?
The term “Ozempic1” likely refers to the original formulation or dosage variant of Ozempic. Regardless of version or strength, all Ozempic products are owned by Novo Nordisk A/S, the same company that developed and patented semaglutide.
Which pharmaceutical company makes Ozempic?
The pharmaceutical company that makes Ozempic is Novo Nordisk A/S, headquartered in Bagsværd, Denmark. Novo Nordisk specializes in diabetes care, obesity treatment, and hormone therapies.
Who manufactures Ozempic?
Novo Nordisk manufactures Ozempic in its own state-of-the-art facilities. Major production sites include Kalundborg and Hillerød in Denmark, Clayton in North Carolina (USA), and Chartres in France. The company maintains strict global quality standards and does not outsource Ozempic manufacturing.
Which company produces Ozempic?
Ozempic is produced by Novo Nordisk A/S. It is the sole company responsible for producing and distributing the medication worldwide.
Is Ozempic owned by Pfizer?
No, Ozempic is not owned by Pfizer. It is owned and manufactured by Novo Nordisk A/S, an entirely separate company based in Denmark.
Is Ozempic made by Eli Lilly?
No, Eli Lilly does not make Ozempic. Eli Lilly produces competing drugs such as Mounjaro (tirzepatide) and Zepbound, which are also used for diabetes and weight management. Ozempic remains exclusive to Novo Nordisk.
Has Elon Musk taken Ozempic?
Elon Musk has publicly stated that he has taken Wegovy, not Ozempic. Both medications contain semaglutide, but Wegovy is approved specifically for weight loss, whereas Ozempic is designed for type 2 diabetes.
Who is the CEO of Ozempic?
Ozempic itself does not have a CEO since it is a brand, not a company. However, its parent company, Novo Nordisk A/S, is led by Maziar “Mike” Doustdar, who became CEO in August 2025. He oversees the company’s operations and the global strategy for products like Ozempic and Wegovy.
Who owns the patent for Ozempic?
The patent for Ozempic is owned by Novo Nordisk A/S. The company holds the intellectual property rights for semaglutide and all its related formulations. These patents are expected to remain active until the early 2030s.
What country is Ozempic from?
Ozempic originates from Denmark. It was developed by Novo Nordisk A/S, a Danish company founded in 1923. The drug was approved first in the United States in 2017 before expanding to Europe and other global markets.

